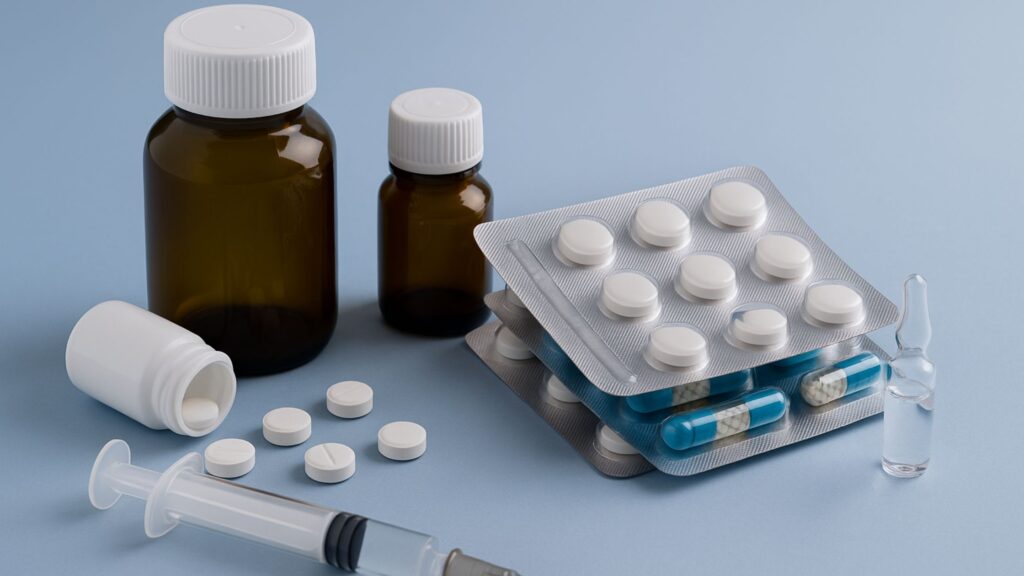
Primary Packaging in Pharmaceutical Industry: The pharmaceutical industry operates in a realm where safety, precision, and quality are paramount. Among the many critical aspects of drug development and delivery, primary packaging in pharmaceutical industry plays a pivotal role. It is more than just a container—it is the first line of defense in protecting the efficacy, integrity, and safety of pharmaceutical products. As the industry continues to evolve with advanced drug formulations, global distribution, and stringent regulatory oversight, understanding the intricacies of primary packaging becomes essential.
In this article, we delve deep into the world of primary packaging in the pharmaceutical industry, exploring its types, importance, materials used, regulatory considerations, and emerging trends.
What is Primary Packaging in Pharmaceutical Industry?
Primary packaging refers to the material that first envelops the drug product, holding it and coming into direct contact with the pharmaceutical formulation. This packaging is essential not only for containing and preserving the medication but also for protecting it from physical damage, contamination, and environmental factors like moisture, light, and oxygen.
Unlike secondary packaging—which typically includes boxes, cartons, or external wrapping—primary packaging has direct interaction with the drug. Therefore, it must meet rigorous safety and compatibility standards.
Importance of Primary Packaging in Pharmaceutical Industry
1. Protection and Preservation
The foremost role of primary packaging in the pharmaceutical industry is to protect the drug from degradation. Many drug formulations are sensitive to environmental conditions, and primary packaging shields them from:
-
Moisture and humidity
-
Oxygen and other gases
-
Light (especially UV)
-
Physical damage and contamination
2. Product Integrity and Safety
Any contamination or chemical interaction between the drug and its packaging can compromise patient safety. Hence, materials used in primary packaging must be inert and non-reactive.
3. Regulatory Compliance
Regulatory agencies like the FDA (U.S. Food and Drug Administration) and EMA (European Medicines Agency) require that pharmaceutical companies demonstrate the suitability and safety of primary packaging materials. The packaging must be shown not to leach harmful substances or degrade the product over its shelf life.
4. Ease of Use and Patient Compliance
Well-designed primary packaging enhances the ease of administration, especially for specific patient groups such as the elderly or children. For example, blister packs and pre-filled syringes provide convenience and dosage accuracy.
5. Tamper Evidence and Anti-Counterfeiting
Primary packaging can incorporate features to show if tampering has occurred. With counterfeit drugs being a global concern, secure packaging is essential for product authentication.
Types of Primary Packaging in Pharmaceutical Industry
The type of primary packaging used depends largely on the dosage form—solid, liquid, semisolid, or injectable—and its specific storage requirements. Common types include:
1. Blister Packs
Blister packaging is one of the most widely used formats for solid oral dosages like tablets and capsules. It typically consists of a thermoformed plastic cavity sealed with a foil or plastic backing.
Advantages:
-
Individual protection for each unit dose
-
Clear visibility of product
-
Tamper-evident features
-
Good barrier properties
2. Bottles
Used for both liquid and solid formulations, bottles are made from glass or plastic and are sealed with caps or closures, sometimes with a tamper-evident seal.
-
Glass bottles: Ideal for sensitive formulations, especially when stability against light and air is crucial.
-
Plastic bottles (HDPE, PET): Lightweight and shatterproof, but may require additional barrier coatings.
3. Ampoules and Vials
Commonly used for injectable formulations, these are glass or plastic containers that are either heat-sealed (ampoules) or sealed with rubber stoppers and aluminum crimps (vials).
Advantages:
-
High sterility assurance
-
Suitable for lyophilized or liquid formulations
-
Excellent barrier properties
4. Pre-filled Syringes
Pre-filled syringes offer a ready-to-use drug delivery system, enhancing patient safety and convenience, especially in emergency or self-administration settings.
5. Tubes
Used for semi-solid formulations like creams, gels, and ointments. Tubes can be made from laminated plastic, aluminum, or composite materials.
6. Inhalers and Nasal Sprays
For respiratory and nasal drug delivery, these devices serve as both primary packaging and delivery systems.
Materials Used in Primary Packaging
Material selection is one of the most critical aspects of primary packaging in the pharmaceutical industry. The material must be chemically compatible, inert, and capable of forming a strong barrier.
1. Glass
-
Type I (Borosilicate): Highly resistant to thermal shock and chemical corrosion; used for injectables.
-
Type II & III (Soda-lime glass): Used for oral solutions and tablets.
2. Plastics
Common polymers include:
-
High-Density Polyethylene (HDPE): Good moisture barrier, used for tablets.
-
Polyethylene Terephthalate (PET): Clear, strong, used for liquid formulations.
-
Polyvinyl Chloride (PVC): Used in blister packs, often combined with aluminum.
3. Rubber (Elastomers)
Rubber stoppers for vials must be biocompatible and tested for extractables and leachables.
4. Aluminum Foil
Often used in blister packs and as a sealing layer due to excellent barrier properties.
Regulatory Aspects of Primary Packaging
Primary packaging in the pharmaceutical industry must comply with a range of regulatory requirements. Key agencies include:
1. FDA (USA)
-
Enforces 21 CFR Part 211, which addresses packaging and labeling controls.
-
Requires full testing of packaging materials including extractables, leachables, and toxicology studies.
2. EMA (Europe)
-
Provides guidance through EudraLex Volume 4, especially Annex 1 for sterile products.
-
Requires documentation in the Common Technical Document (CTD) format.
3. ICH Guidelines
-
ICH Q3D: Focus on elemental impurities from packaging.
-
ICH Q1A: Stability testing under various conditions to evaluate packaging suitability.
Documentation required includes:
-
Certificate of analysis (CoA)
-
Stability data
-
Compatibility studies
-
Extractable/leachable profiles
Emerging Trends and Innovations
The field of primary packaging in the pharmaceutical industry is not static. Rapid innovations are addressing safety, sustainability, and patient-centered design.
1. Smart Packaging
-
Incorporates sensors or indicators to monitor temperature, humidity, or tampering.
-
Can be linked to apps for medication reminders and adherence tracking.
2. Sustainable Materials
-
Growing demand for biodegradable and recyclable packaging materials.
-
Reduction in carbon footprint through lighter packaging and renewable resources.
3. Anti-Counterfeiting Technologies
-
RFID tags, holograms, and unique serialization to track and trace the product.
-
Integration with blockchain technology for supply chain transparency.
4. Customization for Biologics
-
New biologic drugs require specialized packaging solutions due to their sensitivity.
-
Innovations include cryogenic vials and improved barrier films.
Challenges in Primary Packaging
Despite advancements, several challenges persist:
1. Compatibility Issues
Interactions between the drug and packaging materials can compromise stability and safety. Extensive compatibility testing is required.
2. Sterilization Requirements
Maintaining sterility without compromising packaging integrity is a major concern, particularly for injectables.
3. Cost Pressure
Balancing the need for high-quality materials and cost-efficiency remains difficult, especially for generics.
4. Regulatory Complexity
Navigating different regulatory environments across regions requires significant documentation and compliance effort.
Conclusion: Primary Packaging in Pharmaceutical Industry
Primary packaging in pharmaceutical industry is a cornerstone of drug safety, efficacy, and patient compliance. From ensuring the physical and chemical protection of the drug product to meeting global regulatory demands and facilitating user-friendly administration, its significance cannot be overstated.
With rising consumer expectations, stricter regulations, and innovations in drug formulations, the role of primary packaging is evolving. Pharmaceutical companies must invest in robust packaging strategies, materials research, and technology integration to stay competitive and ensure public health.
As the pharmaceutical landscape becomes more complex and interconnected, so too will the demands on primary packaging systems. Embracing this complexity with innovation and foresight is the key to delivering safe, effective, and accessible medicines to the world.
Related Articles
- Secondary Packaging Solutions: Optimizing Your Supply Chain and Product Presentation
- Secondary Packaging of Toothpaste: Everything You Need to Know
- Secondary Packaging of Tea: Materials, Types & Trends Explained
- Secondary Packaging of Shoes: Function, Innovation, and Sustainability
- Secondary Packaging of Shampoo: Purpose, Materials, and Innovations
- Secondary Packaging of Perfume: Purpose, Innovation, and Impact
- Secondary Packaging of Lipstick: Balancing Beauty, Protection, and Sustainability
- Secondary Packaging of Coffee: Complete Guide for Coffee Brands
- Secondary Packaging of Chocolate: The Unsung Hero Behind Every Sweet Success
- Understanding Secondary Packaging of Biscuits: Importance, Types & Benefits