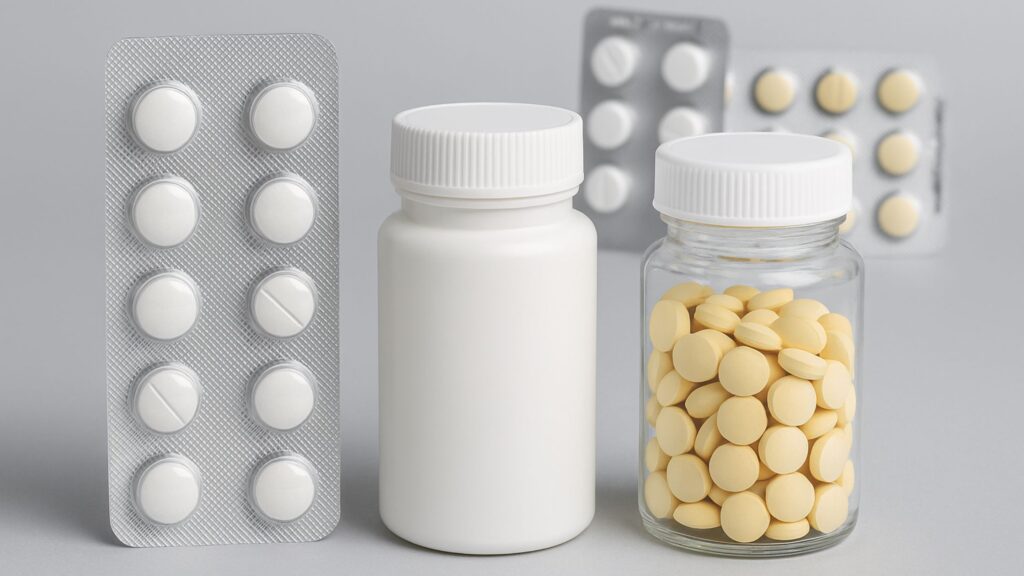
The primary packaging of tablets plays a pivotal role in ensuring the safety, efficacy, and shelf life of pharmaceutical products. While tablets are among the most common dosage forms in the pharmaceutical industry, their effectiveness heavily depends on how well they are protected from environmental factors like moisture, light, and physical damage. In this comprehensive guide, we’ll explore everything you need to know about the primary packaging of tablets—from types and materials to regulatory considerations and innovations.
What Is Primary Packaging?
Primary packaging refers to the first layer of packaging that directly encloses and protects the product. For tablets, this is the immediate packaging that comes into direct contact with the dosage form. It serves not just as a physical barrier, but also plays a crucial role in maintaining the integrity and stability of the product throughout its shelf life.
Unlike secondary or tertiary packaging, which offer external protection or grouping, the primary packaging of tablets is in direct contact with the drug and is thus subject to stringent regulatory and quality requirements.
Importance of Primary Packaging of Tablets
The primary packaging of tablets is essential for several reasons:
-
Protection from Environmental Factors: Moisture, oxygen, light, and temperature variations can degrade active pharmaceutical ingredients (APIs). Packaging protects against these threats.
-
Maintaining Drug Stability: A stable environment helps preserve the chemical and physical integrity of the drug.
-
Patient Safety: Tamper-evident and child-resistant features ensure that the medication is safe to use.
-
Regulatory Compliance: Proper labeling, traceability, and material safety are vital to meet the stringent regulations of agencies like the FDA and EMA.
-
Convenience: Ease of handling, portability, and dose accuracy are critical for patient adherence.
Types of Primary Packaging for Tablets
The primary packaging of tablets is generally categorized into two main formats: blister packs and bottles. Each has its own applications, benefits, and challenges.
1. Blister Packaging
Blister packaging is one of the most widely used forms of primary packaging for tablets. It involves enclosing individual tablets between a formed plastic cavity and a backing material.
Types of Blister Packs:
-
Alu-Alu Blisters: Made entirely of aluminum, these offer the highest protection against moisture, light, and air.
-
PVC/PVDC Blisters: A more cost-effective option, suitable for drugs that are less sensitive to environmental conditions.
-
Cold Formed Blisters: Provide enhanced barrier properties and are commonly used for sensitive drugs.
-
Thermoformed Blisters: These are shaped using heat and pressure and are ideal for less sensitive drugs.
Advantages of Blister Packs:
-
Individual tablet protection
-
Tamper-evident
-
Better patient compliance through unit-dose packaging
-
Easy visual inspection
-
Lightweight and portable
2. Bottle Packaging
Tablet bottles are typically made of high-density polyethylene (HDPE) or polyethylene terephthalate (PET) and closed with tamper-evident screw caps or flip-tops.
Common Bottle Materials:
-
HDPE: High moisture resistance and chemical inertness.
-
PET: Excellent clarity and good barrier properties.
-
Glass: Used for highly reactive or sensitive medications.
Advantages of Bottle Packaging:
-
Ideal for bulk packaging
-
Suitable for large volume distribution
-
Often used in hospitals and pharmacies
-
Can accommodate desiccants and cotton fillers
Key Materials Used in Primary Packaging of Tablets
Choosing the right material for the primary packaging of tablets is critical for maintaining drug quality. The materials must be non-reactive, protective, and compliant with pharmaceutical regulations.
Plastics:
-
PVC (Polyvinyl Chloride): Economical but limited moisture barrier.
-
PVDC (Polyvinylidene Chloride): Enhances moisture barrier when laminated with PVC.
-
PP (Polypropylene): Chemically inert and durable.
-
PE (Polyethylene): Flexible and moisture-resistant.
Aluminum Foil:
-
Used in blister backing for moisture, oxygen, and light barrier.
-
Often combined with heat-seal lacquer for proper sealing.
Glass:
-
Type I borosilicate glass is used for high chemical resistance.
-
Offers excellent protection against gas and moisture.
Regulatory Considerations
The primary packaging of tablets must comply with various regulatory standards to ensure safety and efficacy.
Key Regulatory Bodies:
-
FDA (U.S. Food and Drug Administration)
-
EMA (European Medicines Agency)
-
ICH (International Council for Harmonisation)
-
USP (United States Pharmacopeia)
Requirements:
-
Material Safety: Materials must be non-toxic and suitable for pharmaceutical use.
-
Stability Testing: Packaging must be tested under different environmental conditions.
-
Labeling: Clear, compliant labeling is mandatory.
-
Child-Resistance: Especially for over-the-counter medications.
-
Tamper-Evidence: Packaging must show visible signs if opened.
Innovations in Primary Packaging of Tablets
As the pharmaceutical industry evolves, so does the primary packaging of tablets. Technological innovations are enhancing patient safety, sustainability, and user experience.
Smart Packaging:
-
Integration of NFC chips and QR codes
-
Tracks medication adherence
-
Provides information via smartphones
Sustainable Packaging:
-
Biodegradable films and plastics
-
Recyclable blister packs
-
Reduced carbon footprint in manufacturing
Advanced Barrier Materials:
-
Improved multilayer films
-
Use of nanotechnology for enhanced protection
-
Oxygen scavengers and moisture absorbers
Best Practices for Primary Packaging of Tablets
To ensure quality, safety, and compliance, manufacturers must follow best practices when choosing and handling primary packaging of tablets.
1. Conduct Comprehensive Compatibility Testing
-
Ensure the packaging does not interact with the API or excipients.
-
Test for chemical leachables and extractables.
2. Prioritize Barrier Properties
-
Use high-barrier materials for moisture- and oxygen-sensitive drugs.
-
Consider multi-layer films and foil laminates.
3. Ensure Tamper Evidence and Child Resistance
-
Include features that deter unauthorized access.
-
Ensure compliance with Poison Prevention Packaging Act (PPPA).
4. Perform Stability and Shelf Life Testing
-
Evaluate the impact of packaging on product stability.
-
Use ICH guidelines for long-term and accelerated testing.
5. Focus on User-Centered Design
-
Make packaging intuitive and accessible.
-
Use clear labeling, easy-to-open formats, and patient-friendly designs.
Common Challenges in Primary Packaging of Tablets
Despite advancements, pharmaceutical companies often face challenges with the primary packaging of tablets, including:
-
Material Shortages: Disruptions in the supply chain can affect availability.
-
Regulatory Changes: Frequent updates demand continuous adaptation.
-
Cost Constraints: High-quality materials and testing can be expensive.
-
Counterfeit Prevention: Ensuring product authenticity is a growing concern.
Solutions include diversifying suppliers, using anti-counterfeit technology (like serialization), and investing in R&D for sustainable and affordable materials.
Conclusion
The primary packaging of tablets is far more than just a container—it’s a critical component that ensures the safety, stability, and usability of the medication. With increasing regulatory scrutiny, environmental concerns, and consumer expectations, pharmaceutical companies must invest in innovative, compliant, and patient-friendly primary packaging solutions.
Whether you’re a manufacturer, pharmacist, or healthcare provider, understanding the nuances of primary packaging of tablets will help ensure that the medications you produce, dispense, or prescribe remain safe and effective from production to patient.
Related Articles
- Primary Packaging of Soap: Everything You Need to Know
- Primary Packaging of Shoes: Protecting Style from Factory to Footwear Shelf
- Primary Packaging of Shampoo: Types, Materials & Innovations Explained
- Primary Packaging of Samsung Mobile Phones: Design, Innovation, and Sustainability
- Primary Packaging of Perfume: A Deep Dive into Design, Function & Innovation
- Primary Packaging of Noodles: Materials, Functions, and Innovations
- Primary Packaging of Milk: Materials, Methods, and Innovations
- Primary Packaging of Maggi Noodles: A Deep Dive into Safety, Design, and Sustainability
- Primary Packaging of Lipstick: A Deep Dive into Design, Function, and Innovation
- Primary Packaging of Ice Cream: A Comprehensive Guide